Membrane vesicle trafficking in prokaryotes: molecular biomechanics of biogenesis of outer membrane vesicles of gram negative (Salmonella 3,10:r:-) microbes in chicken ileal invasion model in vivo
(
Introduction & Methods: Human isolate, Salmonella 3,10:...)
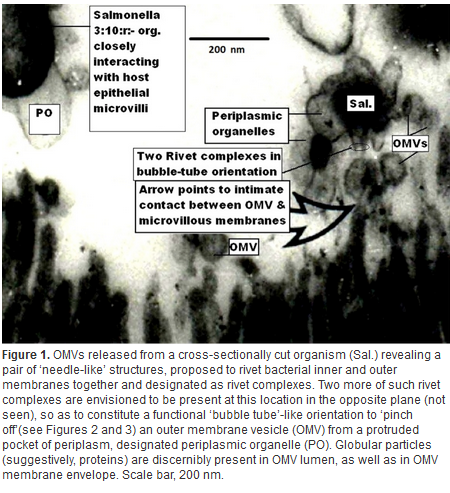
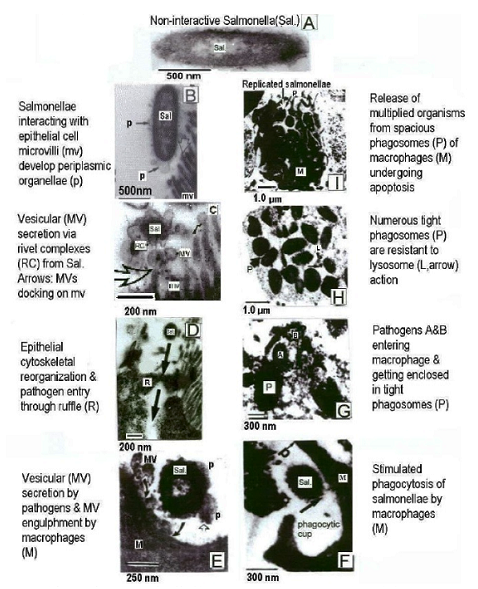
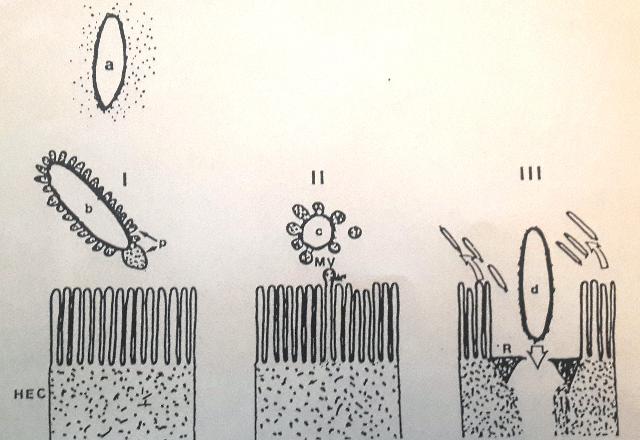
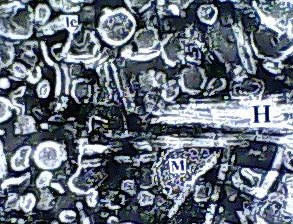
Introduction & Methods: Human isolate, Salmonella 3,10:r:- , known for causing serious food poisoning infections in man, animals and birds was introduced into chicken ileum by standard ligated ileal loop methodology, injecting an aliquot containing 109 cfu, in 24-hr prefasted 3-month old broiler chickens. Eighteen hours later, exudate/fluid that accumulated in the experimental and control loops was drained and ileal tissue pieces suitable fixed. Thereafter, ultrathin sections were examined by transmission electron microscopy to study ultrastructure of biogenesis of bacterial outer membrane vesicles at host-pathogen interface in vivo. Results: Treated ileal sections revealed altered surface ultra-structure of several interactive salmonellae, marked by the presence of numerous pockets or extensions of bacterial periplasm, which also appeared to be liberate 80-90 nm diameter bacterial outer membrane-bound spherical vesicles, in proximity of specialized protruding needle structures embedded in bacterial cell and outer membranes. A molecular biomechanical model for biogenesis of outer membrane vesicles in Salmonella organisms, which may also apply to other gram-negative bacteria, is proposed. This model involves a specialized role assigned to supramolecular protein rivet complexes , suggestively akin to type III secretion nano-machines. RCs are implicated in riveting together, the bacterial inner and outer membranes, thereby, mediating a âbubble-offâ process from pockets of extended bacterial periplasm as OMVs in analogy to soap bubble release with a âbubble tubeâ. At Stage I, heavy secretion of bacterial proteins via well-known general secretory pathway , fills pockets of bacterial periplasm making them protrude out as periplasmic organelles . This is aided by rivet complexes involved in situ riveting of bacterial cell and outer membranes together at the base of POs. Stage II: High concentration of solutes inside POs causes osmotic inflow of water to bring into action a turgor pressure resulting in gradual inflation of POs. Expansion of POs generates a stretch force along the extending bacterial outer membrane at their base, implicated in âpullingâ RCs together, as if âdirectingâ laterally diffusing RCs to mutually align into a bubble tube-like assembly. Stage III: Slight tilting of slender extending needle portions of RCs is allowed by existing smaller diameter of outer hollow rings vis a vis larger inner hollow rings of RCs, coming in physical contact by lateral diffusion. This narrows the âorificeâ at the level of tips of the optimally-extended slender needles, constituting the bubble tube assembly so as to pinch off an OMV from the inflated PO. Discussion & Conclusion: The observed process of trafficking of membrane vesicles from gram-negative micro-organisms to animal host cells is being viewed as a novel cell-cell signaling process, for translocation of bacterial bio-chemicals to animal host or target cells in vivo. A molecular biomechanical model is presented here for OMV biogenesis and discussed in light of other models for this process available in current scientific literature.
http://www.labome.org/research/Membrane-vesicle-trafficking-in-prokaryotes-molecular-biomechanics-of-biogenesis-of-outer-membrane-v.html
2014